2016: A Pivotal Year for Closed Loop/Automated Insulin Delivery
By Adam Brown
 By Adam Brown and Brian Levine
By Adam Brown and Brian Levine
Six systems expected by 2019, plus an FAQ! Which system is right for me?
Want more news like this?
Sign Up Now!
When JDRF started the Artificial Pancreas Project in 2006, the idea of automating insulin delivery using a standard insulin pump, a CGM, and a control algorithm seemed crazy to many patients. Even back in the early 2000s, researchers believed it could be done, but there were many barriers. CGM sensors were not considered accurate enough. Algorithms were immature and untested. Experiments took place in tightly controlled research centers, far from real life. Insulin was thought to be too slow. And there was no clear FDA path to bring an actual product to market.
But a lot can change in ten years, and boy, was 2016 a pivotal year.
In September, the FDA approved the first hybrid closed-loop system for automating insulin delivery to reduce both highs and lows: Medtronic’s MiniMed 670G system including the latest Guardian Sensor 3 CGM. The milestone approval came after an unexpectedly fast three-month FDA review, and just four months after Medtronic reported positive data from its three-month home study. The 670G will launch in spring 2017 in the US, with international approval in some regions expected in summer 2017.
This year also included pivotal updates from at least five other players planning to launch systems in the next few years, including Tandem (launches expected in late 2017 and in 2018), Bigfoot Biomedical (pivotal study in mid-2017), Beta Bionics (insulin-only pivotal study in the second half of 2017), Animas (expected launch in late 2018/early 2019), and Insulet (expected launch in late 2019). We dive into all these systems below in more detail, in order of time to market.
In the meantime, an estimated 100+ people with diabetes globally are not waiting for industry and have built their own do-it-yourself (DIY) automated insulin delivery systems, logging an estimated several hundred thousand real-world “loop hours.” There are now multiple DIY systems in the open-source community, including OpenAPS, Loop, and AndroidAPS. We’ll have a test drive on Loop – which Adam has found very helpful overnight – in early 2017.
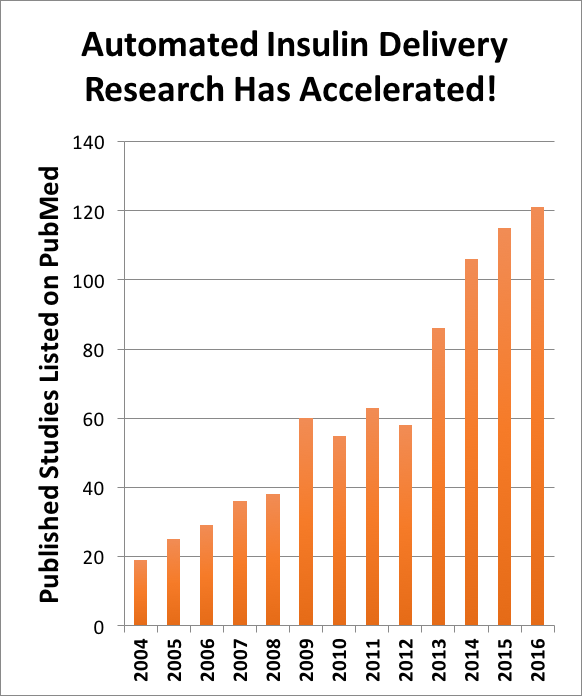 The “closed loop” field started in the academic research setting. A quick literature search shows that there were approximately 20 studies published in 2004, accelerating six-fold to more than 120 this year (see chart).
The “closed loop” field started in the academic research setting. A quick literature search shows that there were approximately 20 studies published in 2004, accelerating six-fold to more than 120 this year (see chart).
We now have a good idea of what to expect from hybrid closed-loop systems that automate insulin delivery:
-
Waking up most mornings with an on-target blood sugar (e.g., 120 mg/dl).
-
More time-in-range and less time spent at extreme high and low blood sugars throughout the day and especially at night.
-
Improvements in A1c for some users with less glucose variability (up-and-down swings).
-
Less diabetes hassle and greater peace of mind for many users, particularly overnight, and especially for parents.
-
Improved sleep quality in many users.
For people with diabetes to benefit from all this research, manufacturers have to incorporate the research into a product, apply for regulatory approval, and then manufacture and sell it. We saw important progress on this front in 2016, with three algorithms originally developed in academia (University of Virginia’s DiAs, Mass General/Boston University’s Bionic Pancreas, and UC Santa Barbara/Harvard) crossing the chasm into companies (Tandem, Beta Bionics, and Insulet) that now plan to bring them to market. The end result, hopefully, is that a number of options will be available in the next few years. Many advocacy organizations, including JDRF, are working very hard on access and cost – if people cannot afford these systems, they won’t really be “available.”
diaTribe has followed this field closely since 2006, and as we sit on the doorstep of real products in 2017, we must thank the visionary leadership of many institutions and people, including the Helmsley Charitable Trust and Trustee Mr. David Panzirer, the JDRF and Chief Mission Officer Dr. Aaron Kowalski, and Mr. Jeffrey Brewer (former JDRF CEO, current Bigfoot Biomedical CEO, and the first person we know to make a major push for more research on this front). We also thank hundreds of relentless investigators and thousands of people with diabetes who have participated in clinical trials. None of the critical research could have taken place without funding from JDRF, the Helmsley Charitable Trust, the NIH, and others, all of whom have played a critical role in advancing automated insulin delivery. Indeed, 2016 was pivotal because these organizations believed in this technology over a decade ago, even when it was far from ready for prime time.
As we prepare for these first and exciting products, many questions and concerns are emerging from people with diabetes:
-
“What do these systems cost?” As noted, access will be critical and value must be proven.
-
“Which option is for me?” Similar to other big purchases, it depends what you’re looking for.
-
“Are these systems truly ‘automated’?” No, they will still need attention and some manual insulin delivery.
See more questions and detailed answers below in our FAQ!
Automated insulin delivery won’t be for everyone with type 1 diabetes, and certainly not at the very start – we hear all the time that it isn’t a cure. That’s true, although data shows very clearly that it will improve glucose levels – many fewer highs and lows – and quality of life in many users, including those with high or low A1cs.
More important, these devices may also save lives. One of the big untold stories in diabetes is death from hypoglycemia overnight (so called “dead in bed”). There are no statistics on how often “dead in bed” happens, but we hear from healthcare providers that it still happens in 2016 – we’re under the impression that there are still dozens of cases a year in the US alone. The progress we’ve seen over the past decade – especially in CGM and now in automating insulin delivery – will hopefully help eliminate these tragic events. “Dead in bed” should never happen, and with the right technology and access, people with diabetes would also never visit the ER due to severe hypoglycemia or DKA. All these events are avoidable with the right management and access to the right technology.
Skeptics would note that pumps and CGMs have been around for years, and they are still used by only about 30% and 10-15% of people with type 1 diabetes in the US, respectively. With that in mind, automated insulin delivery cannot possibly transform diabetes care unless it reaches far more people than pumps and CGMs have in the past. Perhaps automated insulin delivery will be the killer app these two technologies have been waiting for! And hopefully, these new automated systems will be affordable, offer a greater ratio of benefit to hassle, and will be more easily prescribed and taught. It’s a tall order to reach all of that, but 2016 saw a lot of progress and a lot of funding in the field.
Read on for a deep dive into the systems and frequently asked questions about them. Click a question to jump down directly to its answer.
Table of Contents
I. The Players, Products, and Timing – Listed in Chronological Order by Expected Time until Launch
-
Tandem Predictive Low Glucose Suspend and Hybrid Closed Loop
-
Bigfoot Biomedical Smartloop Automated Insulin Delivery Service
II. Frequently Asked Questions
-
Will these systems be “fully automated,” meaning I don’t have to do anything once I press start?
-
Who will benefit the most from wearing automated insulin delivery?
I. The Players
(In order of pivotal study or launch timing, whichever has been shared)
Medtronic MiniMed 670G Hybrid Closed Loop System With Guardian Sensor 3
-
Timing: The FDA approved the 670G in September 2016. Launch is expected in Spring 2017 in the US, with international approval in some regions expected in Summer 2017 (no international launch timing shared). Data from the MiniMed 670G at-home pivotal trial was presented in June.
-
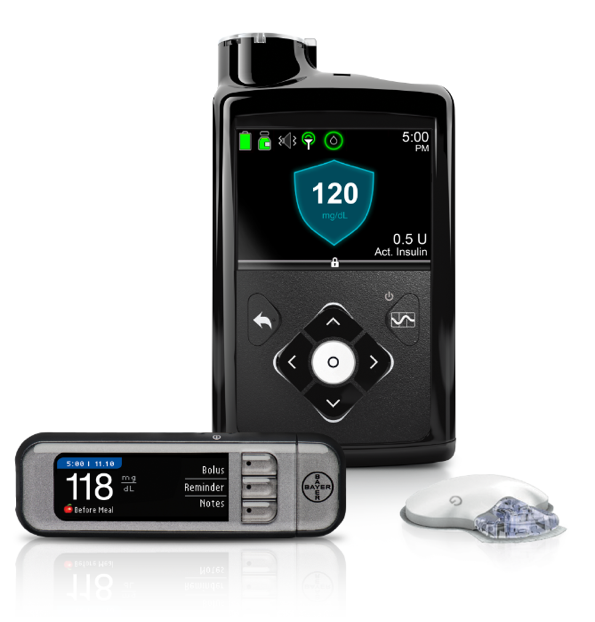 What it will do: Adjust basal insulin delivery every five minutes (based on CGM values) to target a glucose level of 120 mg/dl. If glucose starts trending high, the system may deliver more basal insulin to prevent or limit high blood sugars. If glucose starts going low, it may deliver less basal insulin to prevent or limit hypoglycemia. The target can be temporarily raised to 150 mg/dl during activity. Read more background here.
What it will do: Adjust basal insulin delivery every five minutes (based on CGM values) to target a glucose level of 120 mg/dl. If glucose starts trending high, the system may deliver more basal insulin to prevent or limit high blood sugars. If glucose starts going low, it may deliver less basal insulin to prevent or limit hypoglycemia. The target can be temporarily raised to 150 mg/dl during activity. Read more background here. -
Notable features: Of the 124 people in the pivotal trial, 80% opted to continue using the device through the FDA’s continued access program – a vote of confidence from early adopters. The MiniMed 670G will be the first hybrid closed loop system on the market, and based on the pivotal data, excels at overnight control. The new Guardian Sensor 3 is much more accurate than Medtronic’s previous Enlite CGM in the US. The pump is waterproof and the system also includes a wireless Ascensia Contour Next meter.
-
Keep in Mind: The 670G is only approved for people with type 1 diabetes ages 14 and older, and cannot be used in those under 7 years old and those on less than 8 units of insulin per day. It will not launch with smartphone communication (e.g., remote monitoring or viewing system status/data on a phone), will not issue automatic correction boluses, and the target glucose cannot be changed.
Tandem Predictive Low Glucose Suspend (PLGS) and Hybrid Closed Loop
-
Timing: A pivotal trial of the PLGS system is expected in early 2017, with a launch expected by the end of 2017. The hybrid closed-loop system will enter a pivotal trial in 2017, with a launch expected in 2018.
-
 What it looks like: Both systems will use Tandem’s new t:slim X2 pump, include a control algorithm built into the pump, and talk to a Dexcom CGM. The t:slim X2 with upcoming Dexcom G5 integration (see picture) will serve as the precursor to the PLGS system.
What it looks like: Both systems will use Tandem’s new t:slim X2 pump, include a control algorithm built into the pump, and talk to a Dexcom CGM. The t:slim X2 with upcoming Dexcom G5 integration (see picture) will serve as the precursor to the PLGS system. -
What the systems promise to do: The PLGS system will automatically adjust insulin delivery to minimize the occurrence and duration hypoglycemia. The second-gen hybrid closed loop system will add TypeZero’s treat-to-target algorithm and Dexcom’s G6 CGM, automating insulin delivery to minimize both lows AND highs. See Tandem’s recently published pipeline page here for more details.
-
Notable features: The t:slim X2 is equipped with Bluetooth, which could enable smartphone data viewing/remote monitoring. Tandem’s Device Updater also works with the t:slim X2, meaning remote software updates from home are possible – for example, users could download future closed-loop algorithms without needing a brand new pump. The hybrid closed-loop system will perform automatic correction boluses, further reducing highs over just basal-only systems.
Bigfoot Biomedical Smartloop Automated Insulin Delivery Service
-
Timing: Bigfoot began its first clinical trial in July, with a pivotal trial to start in mid-2017, followed by FDA submission in early 2018.
-
 What it looks like: The service includes: a tubed insulin pump with no screen or buttons and an embedded closed loop control algorithm; a Dexcom CGM; and a smartphone app to view the system status, issue meal alerts, and communicate with the cloud.
What it looks like: The service includes: a tubed insulin pump with no screen or buttons and an embedded closed loop control algorithm; a Dexcom CGM; and a smartphone app to view the system status, issue meal alerts, and communicate with the cloud. -
What the system promises to do: No details have been released on the control algorithm, but we know it will adjust insulin delivery to reduce both highs and lows. If the phone is out of range, the system will remain in closed loop, since the pump contains the algorithm and talks directly to the CGM.
-
Notable features: Bigfoot envisions a subscription model where users access the entire system with one prescription and one monthly price. Bigfoot is also the only upcoming system that will allow a bolus command (“meal announcement”) from a user’s own smartphone. Users will not need to carb count, and instead, will only need to announce that a meal is coming. The user interface for the app is warmer and very different than most medical devices: “Well, hello Sunshine. What’s for breakfast?”
Beta Bionics iLet Bionic Pancreas (insulin-only and insulin + glucagon)
-
Timing: An insulin-only pivotal trial of the iLet is expected to start in the second half of 2017, with an FDA submission expected by mid-2018. An insulin + glucagon pivotal trial is expected in the first half of 2018, though the FDA submission timing is still to be determined.
-
 What it looks like: The third-generation touchscreen iLet pump is pictured, incorporating a closed-loop control algorithm and communicating with a Dexcom G5 CGM. The pump has two separate chambers, one for insulin and one for glucagon, and can operate in insulin-only and insulin + glucagon modes.
What it looks like: The third-generation touchscreen iLet pump is pictured, incorporating a closed-loop control algorithm and communicating with a Dexcom G5 CGM. The pump has two separate chambers, one for insulin and one for glucagon, and can operate in insulin-only and insulin + glucagon modes. -
What the system promises to do: The insulin-only iLet will automatically adjust insulin delivery to reach a user-customizable glucose target (e.g., 100 mg/dl, 130 mg/dl, etc.). The dual-hormone version will add glucagon, bringing additional protection against lows; tighter control without increasing hypoglycemia; and less expected diabetes management burden and greater spontaneity for the user. The insulin + glucagon version will use a custom infusion set with side-by-side tubes for the hormones to flow separately.
-
Notable features: Meal announcement will be optional (both in insulin-only and insulin + glucagon mode). The iLet is the only upcoming system planning to include glucagon, which is expected in a prefilled cartridge. The control algorithm is highly adaptive and only needs body weight to start up, a significant training and setup advantage over other systems. In some markets, Beta Bionics will have prefilled insulin cartridges, in addition to manual fill options. A smartphone app is also under consideration. With glucagon, the iLet will also allow a “G-burst,” enabling a bolus of glucagon to quickly raise blood glucose (e.g., before swimming and disconnecting from the system).
-
Keep in Mind: Beta Bionics’ initial system will be insulin-only. A chronic exposure period for glucagon will be needed in their dual-hormone pivotal trial, making that study longer than the insulin-only trial. No stable liquid glucagon is currently approved, though several are in trials.
Animas Hypoglycemia-Hyperglycemia Minimizer
-
Timing: A launch is expected in late 2018/early 2019. Animas is still working with the FDA to plan a pivotal trial.
-
 What it looks like: It is a control algorithm built into an Animas pump that talks to a Dexcom CGM. Animas showed the following picture at a business update in May, though the product may have changed since that time.
What it looks like: It is a control algorithm built into an Animas pump that talks to a Dexcom CGM. Animas showed the following picture at a business update in May, though the product may have changed since that time. -
What the system promises to do: It will adjust insulin delivery to minimize both highs and lows.
-
Notable features: The pivotal trial is expected to include children as young as two years old.
Insulet Omnipod Horizon
-
Timing: Launch is expected in late 2019, with pivotal trials to follow in late 2018-early 2019. The first in-clinic feasibility study is complete; a second study in pediatric users is currently underway.
-
 What it looks like: It includes: an Omnipod tubeless patch pump worn directly on the body with an integrated control algorithm; a Dexcom CGM; and a Bluetooth-enabled wireless Dash handheld PDM (a locked down Android phone with cellular turned off). The handheld will also talk to a smartphone app to display key data and allow caregivers to remotely monitor.
What it looks like: It includes: an Omnipod tubeless patch pump worn directly on the body with an integrated control algorithm; a Dexcom CGM; and a Bluetooth-enabled wireless Dash handheld PDM (a locked down Android phone with cellular turned off). The handheld will also talk to a smartphone app to display key data and allow caregivers to remotely monitor. -
What the system promises to do: The hybrid closed-loop system will modulate basal insulin delivery. Users will still be expected to give correction boluses and meal announcements.
-
Notable features: The Omnipod is the only tubeless pump available. The hybrid closed-loop algorithm will be embedded in the pod itself, meaning users can stay in closed loop when the handheld is out of range. Pediatric approval is a major priority for Insulet.
-
Keep in Mind: Users will have to use the Dash handheld PDM to issue boluses and interact with the system, though perhaps a smartphone app could take on this functionality over time.
II. Frequently Asked Questions
How expensive are these systems going to be?
There is not a great answer to this question yet, since none of these systems are available yet. These comments below relate to the US.
In most cases, we expect automated insulin delivery systems to be at least as expensive as current pumps and CGMs, or more, but some may be less or some may be made available on “monthly subscription plans.” Without insurance, we imagine automated insulin delivery will be very difficult to afford for most people.
To our knowledge, no US insurance providers have commented on whether they will cover these systems and for whom. We hope that plans see the strong added value of automation for preventing and limiting hypoglycemia (including severe events), keeping glucose in range for more of the day, and improving quality of life. (We hope this also translates into fewer long-term diabetes complications, though this will take time to prove.)
If you currently wear a pump with CGM, the out-of-pocket costs for automated insulin delivery systems may be similar to what you pay now. If you use injections or a pump by itself (without CGM), automated insulin delivery will likely be more expensive than what you pay now. The exact amount will depend on your circumstances, though companies should tell you what you’ll need to pay upfront and on an ongoing basis.
Most systems plan to use a similar pricing model to current pumps and CGMs. [Bigfoot is a possible exception: it plans to have a flat monthly price.] Insurance will typically cover one insulin pump every four years, which usually requires an upfront payment by the insurance company, often with some out-of-pocket cost for the user. The ongoing cost of these systems will include infusion sets, pump reservoirs, CGM sensors and transmitters, and of course, insulin. For those with insurance (particularly high deductible health plans), this can mean a startup out-of-pocket cost in the hundreds or thousands of dollars and ongoing costs in the hundreds or thousands of dollars per year.
For context, the complete MiniMed 670G system – pump, CGM transmitter, and paired glucose meter – will be priced similarly to Medtronic’s current CGM-integrated pumps, ranging from $6,000 - $9,000. Insurance may cover much of this cost if your pump is more than four years old or if you don’t have a pump now, though we’ll have to see what actually happens once the 670G launches. The cash cost to upgrade from current Medtronic pumps (less than four years old) is expensive: $599 or $3,100 out of pocket, including a $400 trade-in credit (depends on whether your old pump was purchased after or before May 1, 2016). Medtronic also has a discount program for Insulet, Tandem, Animas, and Roche in-warranty pumpers to switch to the MiniMed 670G, though it requires paying over $1,000 cash. For those without insurance, Medtronic offers a 20% cash pay discount on the pump and transmitter.
Medicare patients won’t have access to these systems initially, as personal CGM still isn’t a covered benefit. This could change following July’s successful Dexcom FDA advisory committee meeting, which might pave the way for Medicare coverage of Dexcom CGM. Dexcom hopes to obtain Medicare coverage in 2018, just in time for many of these systems to launch. Medtronic has never commented on Medicare coverage of personal CGM.
There is no “correct” answer to this question; like buying a new car, it depends on what you are looking for and what your specific circumstances are. Here are some things to consider with your healthcare provider:
-
Timing and cost – Insurance will typically cover one pump per four years, so if you are on a pump now, your decision may be time-constrained or come with an upgrade fee. Different automated insulin delivery systems may also have different startup costs; we’re not sure how they will stack up until more systems launch. Certain exclusive insurance-pump company arrangements have been announced (e.g., UnitedHealthcare only covers Medtronic pumps for adults) and more may or may not emerge.
-
Current pump and CGM device – It will likely be easier to change from your current pump and CGM to the same company’s system – more device familiarity, less administrative hassle, potentially lower upgrade costs, etc.
-
Interaction, discretion, and burden – Some systems may require interacting with the system only on a pump (e.g., Medtronic’s MiniMed 670G), while others are expected to have wireless communication with a smartphone (Bigfoot) or handheld (Insulet). Each approach has pros and cons: a pump-integrated system like the 670G will minimize the number of devices, while a wireless system may offer more discretion (e.g., less need to pull out the pump in public).
-
Smartphone data viewing and remote monitoring – At this point, we expect at least Bigfoot, Insulet, and Tandem to have smartphone interfaces for viewing data and potentially remote monitoring; we’re not sure about other systems.
-
Tubeless vs. tubed – Insulet’s OmniPod is the only pump currently available that doesn’t require traditional tubed infusion sets.
-
Pediatric approval – If you have a child, check whether the system is approved down to the necessary age. The MiniMed 670G is FDA approved for people with type 1 diabetes ages 14 and over, though a pediatric study (7-13 year olds) is currently underway to investigate lower age groups. Pediatric approval is a big priority for many companies, including Insulet.
-
Algorithm (aggressiveness, customizability, simplicity) – Many algorithms will only change a pump’s basal insulin delivery, though some will add automatic correction boluses (e.g., Tandem’s hybrid closed loop). Some algorithms may be more customizable than others, allowing users to change the aggressiveness for tighter control. Algorithms may also have easier meal announcement options – “small,” “medium,” “large” meal – allowing the system to automatically adapt and eliminating the need to carb count. Beta Bionics plans to have an optional meal announcement.
Will these systems be fully automated, meaning I don’t have to do anything once I press start?
No. Rapid-acting insulin still takes a long time to lower blood glucose (approximately 60-90 minutes to have maximum effect and 3-4 hours to finish working), so even if insulin delivery changes now, glucose won’t respond significantly for 60-90 minutes.
All systems will need user attention and input, since these are first-generation products and won’t work 100% of the time. That means some kind of manual open-loop therapy will be needed when the CGM is not working, and doing infusion set changes every 2-3 days, sensor changes every 7-10 days, reservoir filling, troubleshooting errors, etc.
Users of hybrid closed-loop systems will still need to take manual boluses for meals. (An exception is for meals with very few carbs (e.g., 10 grams), which can often be covered with automated basal insulin alone.) If a bolus is missed, blood glucose will still go high and remain high for some time, even on an automated insulin delivery system. The MiniMed 670G will not give automatic correction boluses, meaning very high blood sugars will need a manual correction bolus to come back down quickly; otherwise, the automated basal insulin will take several hours to bring blood glucose back into range. Other systems may issue a correction bolus automatically if glucose goes too high (e.g., Tandem’s hybrid closed loop), and Beta Bionics will have optional meal announcements.
Will automated insulin delivery reduce my diabetes hassle?
It depends where you are coming from.
For current pump and CGM users, these systems should improve glucose with the same or much lower diabetes hassle (especially overnight).
For injection users, the addition of wearing new device(s) and doing infusion set and sensor changes may add hassle. Every person will have a different view, and for some, taking on more hassle will be worth it for better glucose numbers.
Who will benefit the most from wearing automated insulin delivery?
Those with a high risk of overnight hypoglycemia or hyperglycemia should see significant benefits. Data shows those with low A1c’s and high A1c’s can benefit. Children with type 1 diabetes may experience significant wins, particularly overnight (and by extension, their parents).
What about do-it-yourself (DIY) systems like OpenAPS?
There are now multiple DIY closed loop options in the open source community. Some systems use a tiny dedicated device to bridge communications between a pump and CGM (OpenAPS), while others rely on a small radio bridge and are driven by a phone app (Loop, AndroidAPS, etc.). You can find more details on OpenAPS here, and there is a good overview of the numerous DIY options here. We will return in early 2017 with a detailed test drive of the new Loop app, a DIY system that allows running closed loop from an iPhone. Adam has found it very helpful overnight.
What about people who don’t want to wear an insulin pump? Can algorithms benefit injection users by suggesting changes in insulin doses?
Yes! Several companies are working on taking intelligent algorithms and using them in injection users to suggest changes in insulin doses – this could be called “smarter open loop therapy” or simply “insulin dosing advice.” These algorithms can use either fingerstick or CGM data to suggest changes in injected basal or bolus insulin. For instance, “Based on your glucose patterns, you should take 16 units of Lantus today.” The recommendations update as glucose values change.
Voluntis’ Insulia, cleared by the FDA this month, is a software that will suggest changes in insulin doses for people with type 2 diabetes on basal insulin alone. The system will use fingerstick readings to provide insulin dosing directions, similar to what someone would receive talking to a healthcare provider. The app will launch in 2017 in the US and in Europe. Voluntis also co-developed a similar app called Diabeo for basal-bolus users, which is already available in France. Voluntis does plan to bring the basal-bolus version to the US, but has not shared launch timing.
We expect to see more insulin dose advice software become available in the next few years, either in apps or glucose meters.
Want more news like this? Sign Up Now!
[Photo Credit: Medtronic, Tandem, Bigfoot Biomedical, Beta Bionics, Animas, Insulet]







