Abbott Freestyle Libre 3 Receives FDA Clearance
By Matthew GarzaKatie Mahoney Andrew Briskin
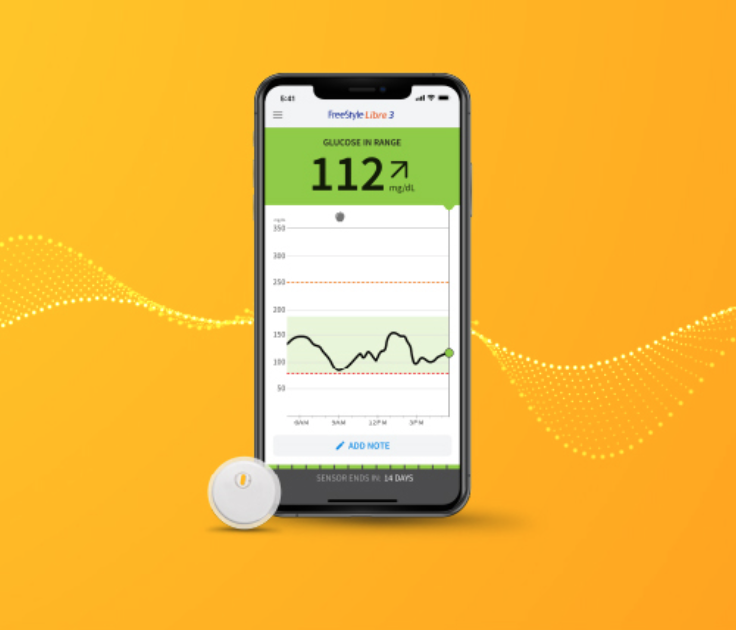
The FreeStyle Libre 3 has been cleared by the FDA for people ages four and older. The new continuous glucose monitor is as small as two stacked US pennies, provides real-time readings directly to the mobile app via Bluetooth, and has the same list price as the Freestyle Libre 2.
On May 31st, 2022, Abbott announced that the new FreeStyle Libre 3 has been cleared by the FDA for people with diabetes in the United States. Libre 3 was "cleared" by the FDA, which means the manufacturer demonstrated that the product was substantially equivalent to similar devices that have already been marketed and received clearance or approval from the FDA.
Freestyle Libre 3 Features
This third-generation continuous glucose monitor (CGM) has many of the same features as the FreeStyle Libre 2, including optional alarms, 14-day wear, and high accuracy. The FreeStyle Libre 3 also adds several new features:
-
Real-time, minute-by-minute readings are sent directly to the FreeStyle Libre 3 app via Bluetooth – unlike the Libre 2, there is no need to manually scan for a glucose reading.
-
It is 70% smaller than previous models, making it the “smallest and thinnest” CGM sensor yet – it’s said to be about the size of two stacked pennies. This will reduce the amount of plastic and carbon paper used, improving device production significantly from an environmental perspective.
-
It is currently the most accurate CGM available.
-
It is cleared for people with diabetes as young as four years old.
-
It is cleared for use in gestational diabetes and pregnant women with type 1 diabetes. We suggest that everyone who is pregnant and has type 1 diabetes try to get CGM, and that everyone else who is pregnant be tested for gestational diabetes as early on as possible.
-
It is cleared as an iCGM, meaning it can be used for automated insulin delivery (AID) development in Europe.
-
The new FreeStyle Libre 3 app, available for both iOS and Android devices, will contain many of the same features as the FreeStyle Libre 2 app (Libre View) including the all-important time in range graphs and ambulatory glucose profile (AGP).
Where is the Freestyle Libre 3 worn?
 The FreeStyle Libre 3 is currently cleared to be worn on the upper-arm. There is no separate reader for collecting and monitoring sensor data, so people will use smartphones with the FreeStyle Libre 3 app in order to connect to the sensor.
The FreeStyle Libre 3 is currently cleared to be worn on the upper-arm. There is no separate reader for collecting and monitoring sensor data, so people will use smartphones with the FreeStyle Libre 3 app in order to connect to the sensor.
How much does the Freestyle Libre 3 cost?
The FreeStyle Libre 3 will be available at the same price, and under the same insurance coverage, as previous versions of the CGM. Abbott will continue to offer the FreeStyle Libre 2 at the same price for people who prefer to scan their CGM.
Where is the Freestyle Libre 3 available?
The FreeStyle Libre 3 launched initially in Germany, with limited availability in the Netherlands. In March 2022, Abbott announced that the FreeStyle Libre 3 would be made available in the UK via the NHS Supply Chain Framework. It appears that the NHS will cover this CGM for everyone with type 1 diabetes and people with type 2 diabetes who are at risk for severe hypoglycemia or who have "unstable blood sugar."
On May 31st, the FDA cleared the Freestyle Libre 3 in the US for people with diabetes ages 4 and up. According to their latest press release, Abbott intends to make the new CGM available at participating pharmacies later in 2022.









