Abbott Receives CE Mark Approval for Its FreeStyle InsuLinx Blood Glucose Meter
Abbott received CE Mark approval for FreeStyle InsuLinx blood glucose meter.
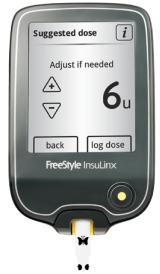
Since receiving CE Mark approval for its FreeStyle InsuLinx in Europe, Abbott has launched the meter in Belgium, France, Germany, the Netherlands, and the United Kingdom. Designed for people with diabetes using mealtime insulin, the meter is Abbott’s first to include a bolus calculator that accounts for fingerstick readings and insulin on board (although the device does not sync up with insulin pumps). Among other data, the InsuLinx stores meter readings, bolus recommendations, pre-/post-meal markers, and carbohydrates. The meter’s USB connectivity allows direct upload to computers and analysis with the FreeStyle Auto-Assist data management software. The Auto-Assist software, which is built into the InsuLinx and is not compatible with previous generations of meters, lets users generate reports and send them to their healthcare providers, and, great news, it is compatible with Mac OS X as well as Microsoft Windows. As a nice touch, InsuLinx users are able to personalize their devices with weekly messages and by uploading a personal photograph. The InsuLinx also features a large, monochrome touch screen, and uses Abbott’s FreeStyle Lite test strips with ZipWik tabs, which make the application of blood easier. Though the InsuLinx is larger than traditional meters, the overall size seems reasonable because the device is thin and light.
In a major win for patients, Abbott has secured reimbursement for the meter in the countries where it was introduced, so it should be available for the same price as the company’s other meters (i.e., FreeStyle, Optium, Precision) for those with coverage. So far, Abbott has not said when it plans to introduce the InsuLinx in the remainder of Europe, nor has the company provided information on the regulatory status of the meter in other regions of the world. --JPS/LV







