ADA Day 1 Highlights – Big Focus on CGM and Access to Diabetes Care
By Jeemin Kwon
 By Jeemin Kwon, Payal Marathe, and Adam Brown
By Jeemin Kwon, Payal Marathe, and Adam Brown
Learn more about DiabetesMine D-Data highlights, implantable CGM Eversense used in adolescents, and Sugar.IQ real-world data
A very good day by ADA standards, the first day of the 78th Scientific Sessions in Orlando offered plenty of thought-provoking presentations and exciting news. Our team was on the ground to absorb all the news, ranging from passionate discussions on the benefits of continuous glucose monitoring (CGM) to a celebration of innovation and patient-centric design.
Key Highlights
1. DiabetesMine D-Data Exchange
-
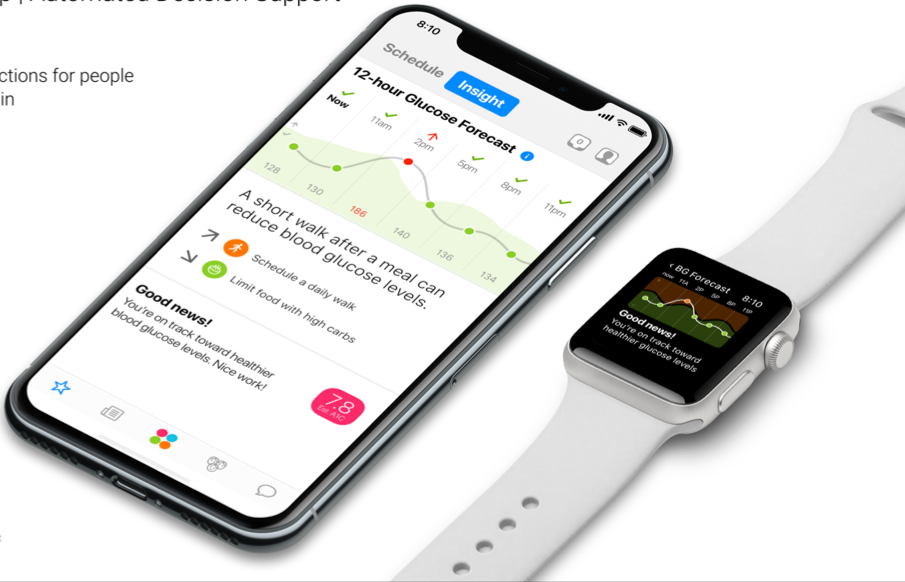
One Drop (a blood glucose meter with coaching) is going to launch an automated decision support feature for type 2 users not on insulin – a forward-looking 12-hour glucose prediction. What this means is that users will be able to see a 12-hour prediction of glucose based on previously logged fingerstick data, food logging, exercise data, and more. This predictive feature will be launched in the second half of 2018. The feature will eventually be updated for people on insulin as well.
-
Ascensia challenge winner: Whisk. Whisk is an artificial-intelligence-powered nutrition platform. Drawing from a massive database, the platform can break down foods’ nutritional content and flavor as well as retailers’ store items and prices. Once a user inputs diet preferences, Whisk makes recipe recommendations and a grocery list and adds the items to an online grocery shopping cart. Whisk will integrate this food platform into Ascensia’s Contour Diabetes app, where blood sugar data will be used to further tailor food recommendations.
-
Pump manufacturer SOOIL, based in Korea, hopes to submit a smartphone-controlled, “open protocol” pump to the FDA “shortly.” The pump appears similar to SOOIL’s Dana RS (not currently available in the US). An open communication protocol will allow the pump to communicate with other devices like CGMs. The company even plans to submit a version of the do-it-yourself (DIY) OpenAPS hybrid closed loop algorithm (for background on closed-loop insulin pump systems, click here). SOOIL’s Dana RS pump is already popular in the DIY community of Europe and Asia because it communicates directly with a smartphone and enables control, including remote boluses, directly from the phone.
2. Continuous Glucose Monitoring
CGM was a big focus during day #1 of ADA – it was encouraging to see the audience’s engagement and to recall that when CGM was first introduced, there were substantial obstacles, such as ease of use, accuracy, and access. The story is quite different today! Of course, CGM is still only used by about 15% of the type 1 population, and even fewer in type 2, but progress has been made. Here are quotable quotes from the day:
-
On factors that impact blood glucose data (which we can now analyze more meaningfully due to CGM): “It is ALL food and lifestyle – we are so excited for the new meds – but I think as we moved into these new medication, we lost sight that diet and exercise have such a huge role in blood sugar control.” – Dr. Irl Hirsch, MD
-
On the potential for CGM to support diabetes management in vulnerable populations: “There’s no significant reduction in A1C among these populations when given CGM. But patients wanted to keep using CGM because they felt better on it. I sense that if we do extra education and have the right tools, we can improve outcomes.” – Dr. Anne Peters, MD
-
On getting CGM coverage from Medicare: “In 2018, reimbursement should not be the thing holding you back from CGM. Now, you can use a phone as a primary receiver or a secondary device – this is great for sharing purposes. So, do not request CGM until the beneficiary has met ALL the coverage criteria. Make sure the info is in there before submitting because if you get denied, then there is a chance you won’t get it in the future.” – Davida Kruger, NP
-
Dexcom vs. Libre?: “If someone has clear hypoglycemia, my preference is Dexcom over Libre because it can alert and has the Share app, which can alert a family member or friend.” – Dr. Irl Hirsch, MD
3. Eversense implantable CGM – first study in adolescents
Senseonics’ Eversense 90-day-wear implantable CGM was recently approved for adults by the FDA. In light of this positive news, we were looking forward to the data on Eversense use in adolescents. Impressively, this study used the 180-day wear sensor available in Europe (Eversense XL) and found the sensor to be consistently accurate throughout. When asked what they liked about Eversense, the participants cited ability to display glucose readings on mobile devices, hypoglycemia/hyperglycemia alerts, predictive hypoglycemia/hyperglycemia alerts, and the sensor duration. They also liked that the sensor is implanted. Senseonics expects to begin recruiting for a US trial of the 180-day Eversense XL this summer.
4. Sugar.IQ – a closer look at the results
diaTribe has previously covered Sugar.IQ, an artificial intelligence-powered companion app to Medtronic’s Guardian Connect CGM – it’s now available in the US for people on iOS devices (find in Apple store here). Today, we learned a bit more about how Sugar.IQ helps users. The combined data from 256 Sugar.IQ and Medtronic MiniMed 530G/Enlite sensor users showed:
-
Time-in-range (70-180 mg/dl) increased by 36 minutes per day
-
Time spent in hyperglycemia (greater than 180 mg/dl) decreased by 30 minutes per day
-
Time spent in hypoglycemia (lower than 70 mg/dl) decreased by 6 minutes per day. The presenter said that decreases in hypoglycemia may have been masked, since users had 530G’s low glucose suspend function at baseline.
We feel that the more tools we have to decrease glucose variability – which translates to feeling better on a daily basis – the better.
These were just our top-of-mind highlights from day 1. Highlights from the rest of the conference are below!
Day 2: Spotlight on DIY Community and Hypoglycemia
Day 3: Emphasis on Add-On Therapies and Better Technology
Day 4: Updates on New DIabetes Therapies







