FDA Approves Farxiga in US for Chronic Heart Failure
By Arvind Sommi
Farxiga (dapagliflozin), a SGLT-2 inhibitor, has been approved by the FDA to treat all types of heart failure, a common diabetes complication.
The FDA has approved Farxiga (dapagliflozin), which is used to lower the risk of cardiovascular death and hospitalizations for adults with heart failure.
The May 2023 decision was based on positive results from the DELIVER clinical trial, which demonstrated that Farxiga has significant benefits for patients with heart failure across the range of ejection fractions (more on that later).
Compared to those who didn't take the medication, people taking Farxiga had approximately an 18% reduced risk for cardiovascular hospitalization and death. Farxiga is now the first SGLT-2 inhibitor approved to treat all types of heart failure.
"The approval of Farxiga in the US not only reinforces AstraZeneca’s commitment to reducing the burden of this complex and life-threatening disease, but will help patients across the full spectrum of heart failure lead healthier lives," Ruud Dobber, executive vice president of AstraZeneca's biopharmaceuticals business unit, said in a press release.
Diabetes and heart failure
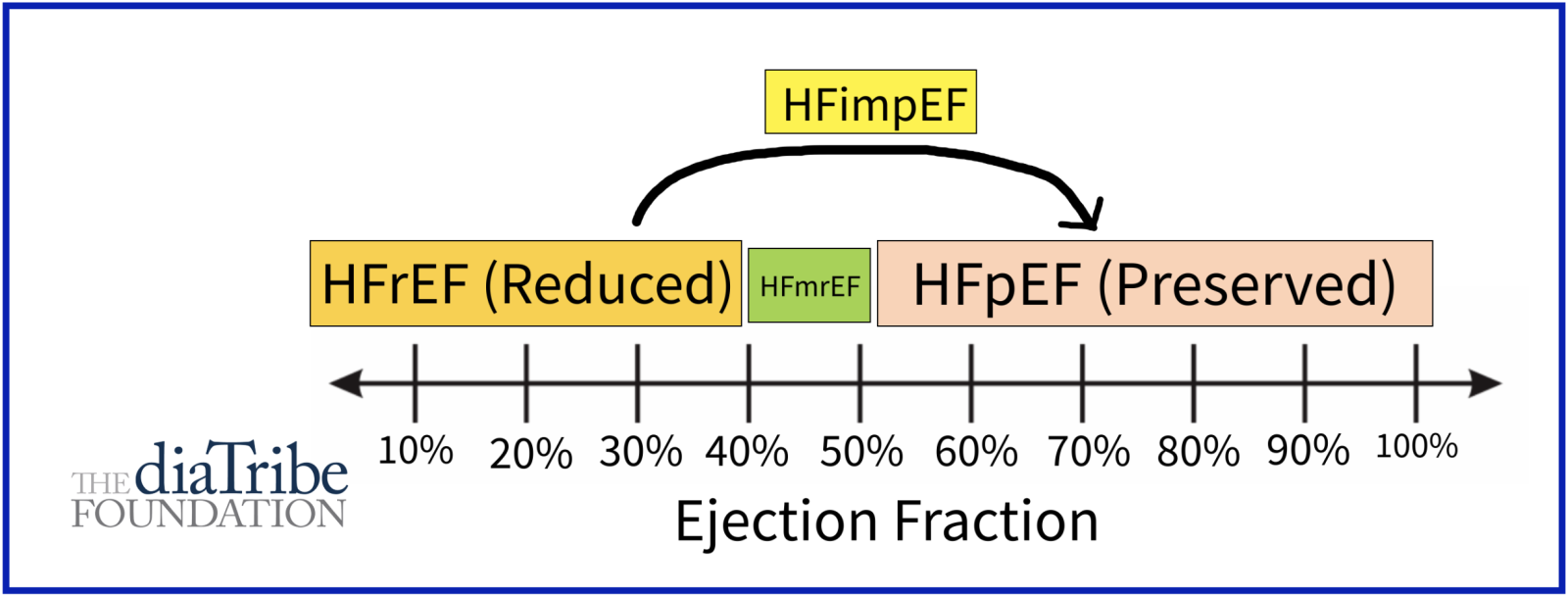
People with type 2 diabetes are 2-4 times more likely to experience heart failure, one of the most common complications of diabetes. Heart failure occurs when the heart cannot pump enough blood to the rest of your body. There are several different types of heart failure, including:
-
Heart failure with reduced ejection fraction (HFrEF): HFrEF occurs when heart muscle becomes weak and can only pump out less than 40% of the blood from the left ventricle, the most important heart chamber.
-
Heart failure with mildly-reduced ejection fraction (HFmrEF): HFmrEF occurs when the heart pumps out between 40% - 50% of the blood from the left ventricle.
-
Heart failure with preserved ejection fraction (HFpEF): HFpEF usually occurs when the left ventricle loses the ability to relax normally because the muscle has become stiff. The heart can't properly fill with blood during the resting period between each heartbeat, However, it can still pump out more than 50% of the blood from the left ventricle. HFpEF is commonly associated with high blood pressure, type 2 diabetes, obesity, and kidney disease.
-
Heart failure with improved ejection fraction (HFimpEF): HFimpEF occurs when cardiac function increases with treatment from HFrEF to HFmrEF or HFpEF.
Why is the DELIVER trial important?
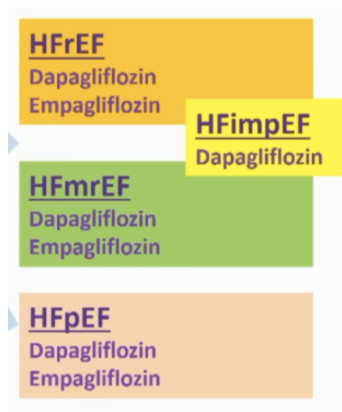
The DELIVER trial included participants with heart failure who did not have a reduced left ventricular ejection fraction (LVEF ≤40%; HFrEF), including those with and without type 2 diabetes.
The trial confirms the previous findings of the EMPEROR-Preserved trial that found another SGLT-2 inhibitor, empagliflozin (Jardiance), achieved similar results in this group of participants with heart failure.
However, DELIVER is unique because it included participants with additional types of heart failure, and together with earlier trials, showed improvement for participants with any type of heart failure – with or without diabetes.
Additionally, the DELIVER trial results were consistent in participants from outpatient settings and those enrolled during or within 30 days of hospitalization. These findings suggest many people with diabetes and heart failure, whether or not they have diabetes, should consider starting a SGLT-2 inhibitor, like Farxiga, immediately upon diagnosis of heart failure, including while in the hospital – even before the ejection fraction is measured since it is effective in any type of heart failure regardless of cardiac function.
While Farxiga has been approved to treat those living with chronic kidney disease, HFrEF, and type 2 diabetes, it is not recommended for people with type 1 diabetes because it can increase the risk for a serious health complication, diabetic ketoacidosis.
To learn more about heart failure, read our other articles:








