LifeScan’s OneTouch VerioSync Blood Glucose Meter Cleared by FDA
By Adam Brown

On February 7, the FDA cleared LifeScan’s OneTouch VerioSync blood glucose meter. It will feature wireless Bluetooth compatibility with the iPhone, allowing blood glucose readings taken on the meter to be sent wirelessly to an iPhone app (see picture at left). LifeScan has not publicly announced the FDA clearance or stated when the new meter might become available in the US. We first learned about the positive regulatory decision on the FDA’s website (both the meter and the software application were cleared), as the agency posts records of all medical devices that are cleared and approved.
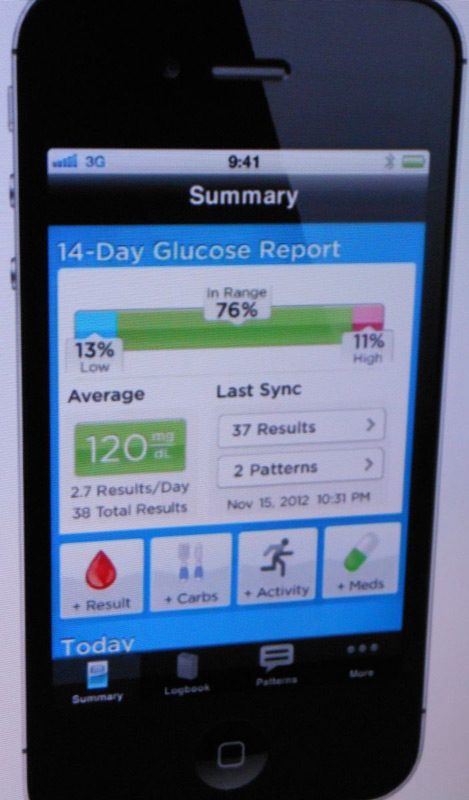
As noted in diaTribe #46, we got a sneak peek at the OneTouch VerioSync at the American Association of Diabetes Educators Annual Meeting last August. The VerioSync makes data uploading hassle-free, a trend seen in other glucose monitoring products as well (e.g., Telcare’s meter, Sanofi’s iBGStar, and Glooko’s MeterSync cable). Our favorite part of the VerioSync iPhone app is the summary display (see picture at right), which prominently shows time-in-range and average blood sugar. These statistics can be motivating and useful – especially time-in-range for identifying problem areas – and it’s good to see them front and center on the sleek-looking iPhone app. As we described in our test drive on the OneTouch Verio IQ, the Verio platform has the ability to automatically recognize patterns of high and low blood glucose. Based on the screenshots we’ve seen, this feature will also be part of the new VerioSync. In short, this is a meter we’re excited to try – stay tuned for our test drive! –AB







