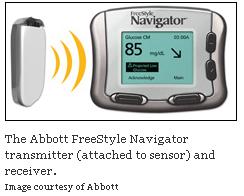
Navigator Approval

New data from a large clinical trial of adults who had experienced a recent heart attack showed that the SGLT-2 inhibitor Jardiance reduced the risk of...
Explore 'exercise snacks,' short bursts of activity that can aid diabetes management, improve heart health, and fits busy lifestyles.
You notice there’s a buzz around...
Charcot foot is an uncommon, but...
Explore GLP-1 agonists for weight loss in type 1 diabetes with caution. Understand potential risks like hypoglycemia and DKA, while balancing benefits for heart and...
Due to increased demand, diabetes and weight loss medications like Mounjaro, Ozempic, Wegovy, and Zepbound continue to be in short supply for the foreseeable future....