New Tech Advancements Make Insulin Delivery Easier for People with Diabetes in Europe
 By Eliza Skoler and Albert Cai
By Eliza Skoler and Albert Cai
Two diabetes devices from Medtronic – a next-gen continuous glucose monitor that removes fingerstick calibrations and a smart insulin delivery pen that keeps track of your insulin doses – will become available in Europe later this year. The two can share data with one another to simplify diabetes management for people on multiple daily injections of insulin.
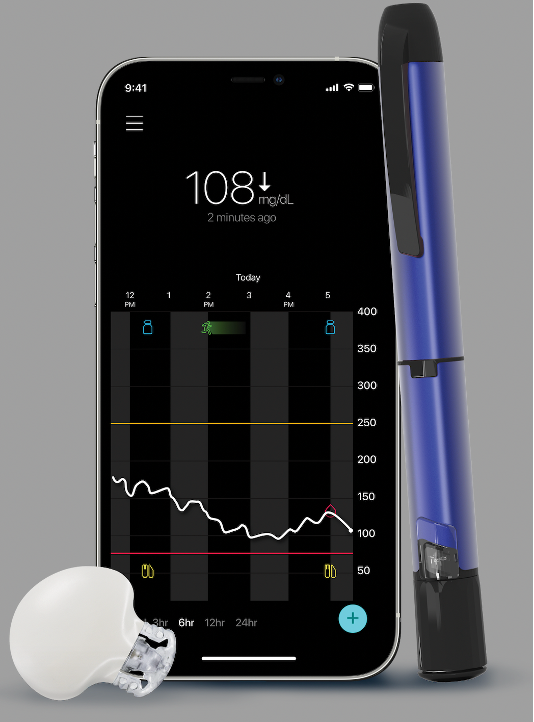
Medtronic recently announced that the Guardian 4 continuous glucose monitor (CGM) and its InPen smart insulin pen will launch this fall in Europe. Both devices have been approved in Europe for people with diabetes. The two will be integrate with one another through real-time data sharing, to help people on multiple daily injections of insulin (MDI) track and calculate insulin doses more easily.
Medtronic’s Guardian 4 is the company’s first CGM that does not require fingerstick calibrations. Guardian 4 has the same shape and seven-day wear as Medtronic’s existing Guardian 3 CGM system, but does not need fingerstick calibrations and users can bolus insulin based on CGM readings alone, without having to perform a confirmatory fingerstick. Both Guardian 3 and 4 sensors have a two-hour warm-up time period, offer customizable predictive low and high glucose alerts, and have an alarm for urgent lows set at 54 mg/dL. In addition to InPen, Guardian 4 integrates with Medtronic’s MiniMed 780G Advanced Hybrid Closed Loop system without the need for fingersticks (and including the new seven-day infusion set), which rolled out in Europe in fall 2020. In the US, both Guardian 4 and Medtronic MiniMed 780G have been submitted to the FDA for approval but are not yet available.
InPen is a one-year, reusable, Bluetooth-enabled smart insulin pen. It automatically captures mealtime doses and keeps track of insulin on board (how much insulin is still active inside your body from the previous bolus). The InPen smartphone app (available for iOS and Android) tracks insulin doses and glucose levels, includes a bolus calculator, and creates an InPen Insights report that combines glucose and insulin data for review including your previous data. These reports are great to review with your healthcare team to help inform your diabetes management. InPen is the only smart insulin pen approved in the US or Europe that integrates with real-time CGM data – in the US, the app currently works with Medtronic’s Guardian Connect CGM.
What else is coming from Medtronic?
Medtronic is working to combine technologies across CGM, insulin delivery, and artificial intelligence to “close the loop” for people using MDI. Specifically, the company aims to combine InPen with meal detection software (The Nutrino software can identify what food is being consumed, and the Klue software can identify when food is being consumed) to make it easier for people to determine their optimal mealtime insulin dose without having to count carbs. This could help simplify diabetes management for people on MDI, providing some of the benefits of automated insulin delivery (AID) systems to those who are not using insulin pumps.
Medtronic is also planning to submit its next-generation, fully disposable CGM, “Synergy,” to the FDA later this summer. Synergy will have a smaller on body footprint – it will be 50% smaller than the Guardian 3 and 4 sensors – and will include seven-day wear-time and self-calibration (meaning no fingersticks).







