June and July have been very busy months in the diabetes world...
Type 2 Diabetes News
Type 2 Diabetes is a chronic condition involving high blood sugar levels and reduced production and responsiveness to insulin.
61 readers recommended
Exciting findings from the largest diabetes meeting of the year, the American Diabetes Association’s annual Scientific Sessions.
34 readers recommended
Allergan’s LAP-BAND, an implantable weight-loss device. Following an FDA advisory committee ’s recommendation last December (see New Now Next in diaTribe #28), the FDA...
25 readers recommended
We were heartened this year by World Diabetes Day, which officially recognizes the growing epidemics of diabetes, diabetes complications, and obesity. Every year on...
42 readers recommended
Last week, in a surprise and, in our view, disappointing move, the FDA decided to not approve Bydureon, a once-weekly GLP-1 agonist, for use in the United States despite...
47 readers recommended
Imagine dreaming about waking up alive inside a body bag. Vietnam veteran Urban Miyares did, but it wasn’t exactly a dream...
37 readers recommended
Asante’s Pearl insulin pump has received FDA clearance and CE Mark approval. In welcome news, Asante Solutions received FDA clearance for its new Pearl insulin pump in...
39 readers recommended
Even though exercise and weight loss have been shown to reduce a person’s chance of developing type 2 diabetes, healthcare providers have not traditionally reimbursed...
32 readers recommended
Stellar diabetes experts descend on San Francisco for the ADA’s 57th Annual Advanced Postgraduate Course.
43 readers recommended
The LAP-BAND is a weight-loss device that is implanted around the top of the stomach using minimally invasive (laparoscopic) surgery, which effectively creates a small...
69 readers recommended
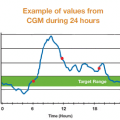
Kerri Morrone Sparling gives her take on the pros and cons of CGM.
48 readers recommended
Puzzled by the recent FDA decisions? Dr. Sanjay Kaul discusses the regulatory environment for diabetes and obesity medications.
39 readers recommended
No doubt, you have in all likelihood heard about the FDA’s decision to restrict access to Avandia, based on data suggesting its use is associated with an elevated risk...
42 readers recommended
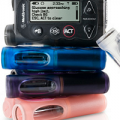
An evolutionary step for Paradigm pumpers – see Dana Lewis’s take on Medtronic’s new pump/CGM integrated platform the Paradigm REAL-Time Revel system.
34 readers recommended
We discuss the “motivated patient” and highlights from the American Association of Diabetes Educators’ annual meeting as well as big changes here at diaTribe …
28 readers recommended
While we’re willing to bet that most people with diabetes think that blood glucose meters are an important tool, there is surprisingly little scientific evidence...
37 readers recommended
The FDA just approved Medtronic’s latest insulin pump, called the MiniMed Paradigm REAL-Time Revel System – this is Medtronic’s first new pump platform to be approved in...
49 readers recommended
A thorough review and “how-to” on the drug Symlin (Amylin’s pramlintide).
43 readers recommended
Earlier this week, the FDA chose not to approve the weight-loss drug Qnexa. Qnexa is a combination of two currently approved drugs: phentermine, which is approved for...
68 readers recommended
Our top takeaways from the European Association for the Study of Diabetes meeting in Stockholm, and The Obesity Society meeting in San Diego.