Summer 2021 DiabetesMine D-Data ExChange
By Talia PikounisArmaan Nallicheri
 At the third virtual DiabetesMine D-Data ExChange, we learned about the latest in DIY automated insulin delivery and the newest in diabetes technology from smart insulin pen caps and minimally invasive continuous glucose monitors.
At the third virtual DiabetesMine D-Data ExChange, we learned about the latest in DIY automated insulin delivery and the newest in diabetes technology from smart insulin pen caps and minimally invasive continuous glucose monitors.
The June 2021 DiabetesMine D-Data Exchange gathering of leaders in diabetes technology and innovation focused on new information about DIY automated delivery systems (AID) for people with diabetes who take insulin, and showcased new technology options, such as Bigfoot’s Unity System, Waveform’s needle free and minimally invasive Continuous Glucose Monitor (CGM), and Level’s app-based wellness program.
Last year’s D-Data event highlighted how the DIY community was advancing AID systems. This year, Katarina Braune (Charité University Medicine Berlin) presented findings from the OPEN Diabetes Project, an international collaborative group led by people with diabetes, on decision-making around DIY AID systems, lived experiences, and barriers to use.
The collaborative group collects data from both adults and children living with diabetes. In one real-world study presented by Dr. Braune, a group of 365 people with diabetes were included. Before using the DIY technology, the participants had an average A1C of 7.1% and spent around 63% of their day in Range. The study found that DIY AID systems helped people with diabetes achieve better control over their blood glucose.
-
Among both adults and children, Time in Range increased to 80% (an increase of 4.2 hours/day) after starting the open-source AID systems
-
In addition, their average A1C dropped 0.9 percentage points to 6.2%.
The OPEN Diabetes Project has also conducted research recently on people’s reasons for using or not using these AID systems.
-
Data from 897 people with diabetes (722 adults and 175 children) found this technology also led to improvements in sleep and gaining back time.
-
In another study, 648 people (519 users and 129 non-users) with diabetes reported not using DIY AID systems primarily due to difficulties obtaining the pieces needed and feeling a lack of support from their healthcare professionals. Although the cost of the necessary components was cited as a barrier in this group, the study did not find any “prominent” differences in income in people who chose to use this technology.
Bigfoot Unity System
DIY AID was not the only interesting topic presented on at the D-Data event. The Bigfoot Unity system was another hot topic. Dr. Jim Malone presented on this system, which the FDA approved just this past May for people 12 years or older with type 1 or type 2 diabetes; it includes two smart caps that work with disposable insulin pens. These caps can act as a FreeStyle Libre scanner (the white pen cap) and can gather information from a CGM to help recommend insulin doses.
The system currently is only compatible with whole unit insulin pens, but Bigfoot is working to make this technology usable with the half-unit pens that many children with diabetes use. You can find a list of all compatible pens here.
Waveform’s CGM
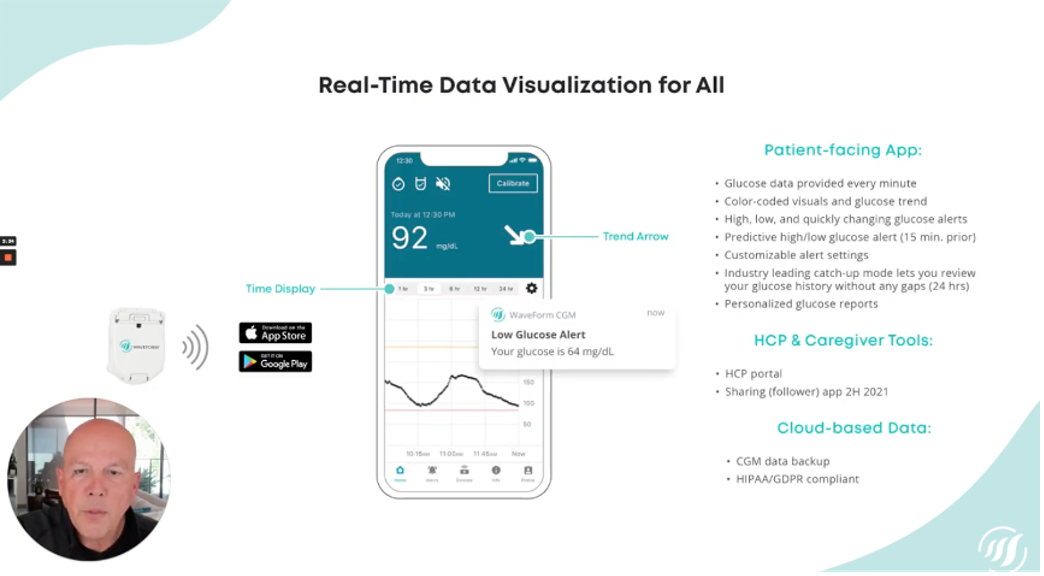 Up next, Rick Valencia from Waveform delivered a presentation on its CGM, which is currently only available in Europe under the name GlucoMen Day. This minimally invasive CGM can be worn for 15-days, offers customizable alerts similar to other CGMs (such as notifying you 15-minutes before your glucose might be high or low, or telling you when your blood sugar is rapidly changing), and sends minute-by-minute data directedly to a connected app. The system uses a small wire under the skin to measure interstitial sugar levels – similar to other CGMs – but it can be inserted without the use of the needle (leading to less pain and leading to longer wear time).
Up next, Rick Valencia from Waveform delivered a presentation on its CGM, which is currently only available in Europe under the name GlucoMen Day. This minimally invasive CGM can be worn for 15-days, offers customizable alerts similar to other CGMs (such as notifying you 15-minutes before your glucose might be high or low, or telling you when your blood sugar is rapidly changing), and sends minute-by-minute data directedly to a connected app. The system uses a small wire under the skin to measure interstitial sugar levels – similar to other CGMs – but it can be inserted without the use of the needle (leading to less pain and leading to longer wear time).
-
The system does currently require one calibration every day, but Waveform is working to reduce the number of necessary calibrations down to once every two to three days.
-
Waveform is also working on extending the wear time of the device, aiming for a 21-day sensor wear time.
-
Though the product is currently only available in Europe, Waveform announced that it is seeking FDA approval in the U.S. hopefully by 2023. There is already a pre-pivotal trial underway and a second trial is set to begin later in 2021.
Level’s app-based wellness program
 Dr. Casey Means presented on another program of interest – the new app from Level which helps interpret your glucose information from CGM readings and then recommends ways to improve your diet and lifestyle based on that data. Using your daily glucose trends, the app suggests how eating a certain food could lead to spikes in your sugar or help keep you around your target glucose level. The app also enables users to upload food photos to keep track of diet, and it tracks sleep and activity to provide insights on how these actions impact blood sugar as well. The technology is still in the testing phases; it appears to use Abbott Freestyle sensors, and is presumably used off-label.
Dr. Casey Means presented on another program of interest – the new app from Level which helps interpret your glucose information from CGM readings and then recommends ways to improve your diet and lifestyle based on that data. Using your daily glucose trends, the app suggests how eating a certain food could lead to spikes in your sugar or help keep you around your target glucose level. The app also enables users to upload food photos to keep track of diet, and it tracks sleep and activity to provide insights on how these actions impact blood sugar as well. The technology is still in the testing phases; it appears to use Abbott Freestyle sensors, and is presumably used off-label.







