Top Highlights: Day 1 of ADA Scientific Sessions
By Eliza SkolerJimmy McDermott
 By Eliza Skoler, Jimmy McDermott, Matthew Garza, Divya Gopisetty, Frida Velcani, and Karena Yan
By Eliza Skoler, Jimmy McDermott, Matthew Garza, Divya Gopisetty, Frida Velcani, and Karena Yan
We bring you the most exciting news from day one, including updates on automated insulin delivery, ways that technology can reduce hypoglycemia, and new physical activity recommendations
ADA’s 80th Scientific Sessions is off to a great start. Here are some of our most interesting learnings from today’s virtual sessions.
Click to jump down to a section:
-
Is Technology the Solution to Hypoglycemia? Dr. Bergenstal and Dr. Wilmot Debate
-
New Physical Activity Recommendations for Adults and Children
The Next Generation of Automated Insulin Delivery Systems for People with Type 1 Diabetes – Updates from Four New Clinical Trials
The first day of ADA featured data on four clinical trials of the newest automated insulin delivery (AID) systems. In what was a packed (virtual) room, the session began with three highly anticipated presentations of studies on Medtronic’s MiniMed 780G Advanced Hybrid Closed Loop System (AHCL). Dr. Bruce Bode, presented the US adult pivotal trial. Here are the main results:
-
Big news – nearly 80% of participants achieved a time in range of more than 70% without an increase in hypoglycemia.
-
On average, AHCL therapy increased time in range to nearly 75% from a baseline of 68.8%.
-
Among adolescents, time in range increased to over 72% from a baseline of 62.4%.
-
-
AHCL therapy improved average A1C from 7.5% to 7.0%. This is what is sometimes called a “high quality A1C” in the field – hypoglycemia is low, and therefore not contributing to a “better” number.
-
How were these results achieved? Experts said that the lower algorithm target of 100 mg/dl (vs. 120 mg/dl) helped, along with an active insulin time (AIT) setting of 2-3 hours. If you use a pump, check what you have for this setting and talk to your healthcare professional about it to see if you can make changes (regardless of whether your pump can deliver insulin automatically).
Following Dr. Bode, International Diabetes Center’s Dr. Rich Bergenstal shared data from FLAIR, a trial comparing MiniMed 780G Advanced Hybrid Closed Loop (AHCL) with the 670G Hybrid Closed Loop (HCL) in adolescents and youth with type 1 diabetes (ages 14-29). This is the first ever head-to-head comparison of an AID system with a commercially available AID system. The study also had broad entry criteria: at start, 20% of participants were on multiple daily injections of insulin (MDI), 38% were not using CGM, and 25% had a baseline A1C above 8.5%.
-
Time in range over 24 hours increased from 57% at baseline to 63% with the 670G and to 67% with the 780G. Notably, 6% greater time in range totals nearly an hour and a half more time in range per day.
-
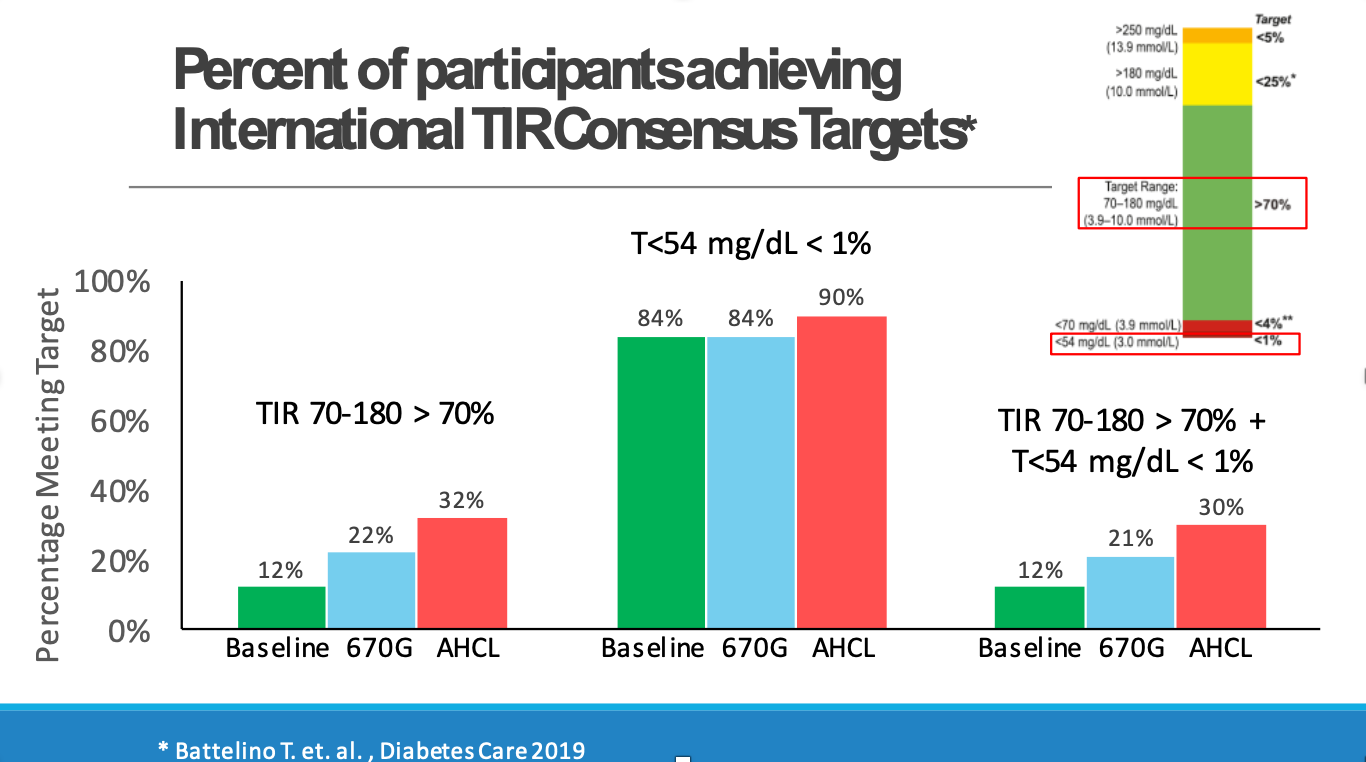
Compared to baseline, the number of participants achieving the international time in range consensus target of more than 70% was nearly two times higher with the 670G and almost three times higher with the 780G (22% and 32% of participants, respectively, compared to a baseline of 12%; see slide below).
-
This was the first time that a study measured participants meeting the combined metric of both time in range greater than 70% and time below 54 mg/dL less than 1% (see slide below). This is important since all therapy – and particulary automated insulin delivery – aims to decrease hyperglycemia and hypoglycemia.
-
From a baseline average of 7.9%, those on the 670G achieved an average A1C of 7.6%, and those on the 780G had A1Cs that fell to 7.4% on average.
-
Both the 670G and 780G were considered safe when evaluating severe hypoglycemia or diabetic ketoacidosis (DKA).
-
Participants satisfaction favored the 780G over the 670G.
Today’s MiniMed 780G data finished with Dr. Martin de Bock’s study, which served as the clinical trial supporting 780G’s CE-Mark submission (and today’s announced approval in Europe). In a study of 59 people (ages 7-80 years, with an average age of 23) who had never used an insulin pump:
-
Average time in range increased to over 70% from 58% (a change of 12.5%) when using the 780G compared to a sensor augmented pump.
-
Overnight time in range increased to 75% from 59% when using the 780G compared to the sensor augmented pump.
-
The improvement in time in range was primarily driven by a 12.1% decrease in time in hyperglycemia (high blood sugar) with the 780G.
It was warming on Twitter to see Dr. de Bock with his three small children while also engaging in Q&A/Chat from their breakfast table. If you’re on social media, follow Dr. De Bock here.
The session concluded with Stanford’s Dr. Bruce Buckingham who presented data on Insulet’s Omnipod 5 Automated Glucose Control System, powered by Horizon. What fantastic data! The study assessed the safety and effectiveness of the fully on-body system over 14 days of use before starting the three-month pivotal study. Interestingly, this study was conducted during the winter holiday season when some of the lowest time in range is observed (typically a three percent drop); the system performed remarkably well in both children and adults, even during this challenging time period.
-
In adults, time in range increased to 73% on the hybrid closed loop system, up from 65.6% using standard therapy – this is the same as nearly two hours more time in range per day.
-
In youth, time in range increased to 70% on the hybrid closed loop system, up from 51% using standard therapy – what an increase, nearly five hours more per day.
These reductions in time in range were mostly driven by a decrease in hyperglycemia. Hypoglycemia was also very low to start. Dr. Buckingham eloquently emphasized, “… this is so important for families and people at night to go to sleep and not worry about hypoglycemia … for a number of kids, they got to go on their first sleepover during this study. It was really decreasing a lot of the burden and a lot of the thinking about diabetes.”
Is Technology the Solution to Hypoglycemia? Dr. Bergenstal and Dr. Wilmot Debate
Dr. Richard Bergenstal from the International Diabetes Center (IDC) emphasized the advantages of using continuous glucose monitoring (CGM) for reducing episodes of hypoglycemia (low blood sugar) and other health complications in this debate with Dr. Wilmot. Both doctors are highly regarded, and we took this as a big opportunity to learn lots more rather than land only on one size, though it’s certainly hard to avoid saying yes to this question, from diaTribe’s perspective. Dr. Bergenstal eloquently explained that, on average, hypoglycemia is the biggest barrier to optimal blood glucose management, pointing to the fact that A1C levels increase when people fear going low (what he called the "ripple effect of hypoglycemia"). Luckily, with CGM reports, people can finally detect patterns in hypoglycemia and understand exactly how much time they are spending with blood glucose levels under 70 mg/dL in a day.
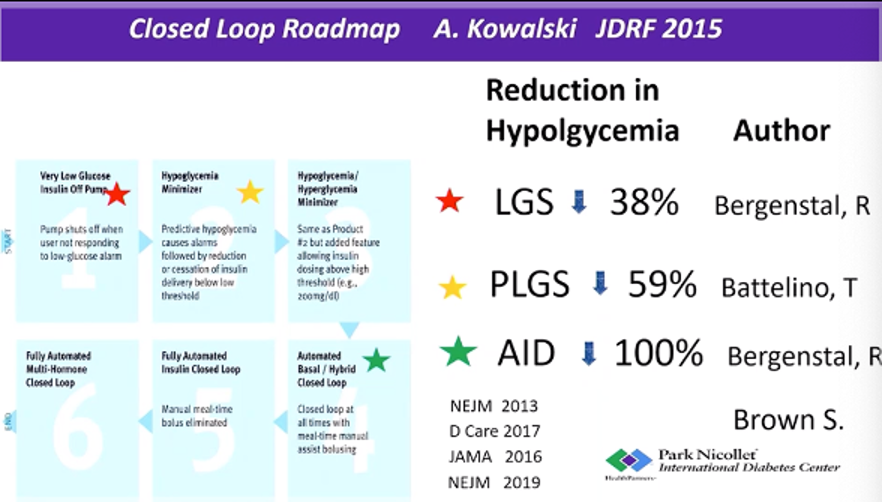
Evidence shows that closed-loop technology can reduce and even prevent hypoglycemia. In a study of 124 people with diabetes that Dr. Bergenstal shared, the use of automated-insulin delivery systems (AID) completely eliminated hypoglycemia. This was a historic win – previous studies (see slide below) using low glucose suspend systems (LGS) reduced hypoglycemia by 38%, while predictive low glucose suspend systems (PLGS) reduced hypoglycemia by 59%.
Dr. Emma Wilmot argued that while these findings are exciting, technology is only part of the solution. Technology does reduce the risk of hypoglycemia, but is not available to all (particularly those from underserved populations) and is not suited to all. She said that unless CGM is also paired with structured education, it will not provide the significant and lasting improvements in hypoglycemia awareness that the diabetes community needs. We know, of course, how important education is – and diaTribe will be coming back to discuss this in an upcoming piece about a new article just published in Diabetes Care earlier this week (Diabetes Sisters’ CEO Anna Norton was a key author in the new consensus report)!
New Physical Activity Recommendations for Adults and Children
Dr. Katrina Piercy and Dr. Ronald Sigal presented the 2018 Physical Activity Guidelines for Americans, with updates to the age-specific guidelines and evidence of even more health benefits. These are the recommendations for each age group:
-
Children ages 3-5 should be physically active throughout the day to support their growth, development, and motor skills. Though the US guidelines do not include a specific amount of time, Australia, the United Kingdom, and Canada recommend three hours per day.
-
Children ages 6-17 should do at least 60 minutes a day of moderate or vigorous physical activity.
-
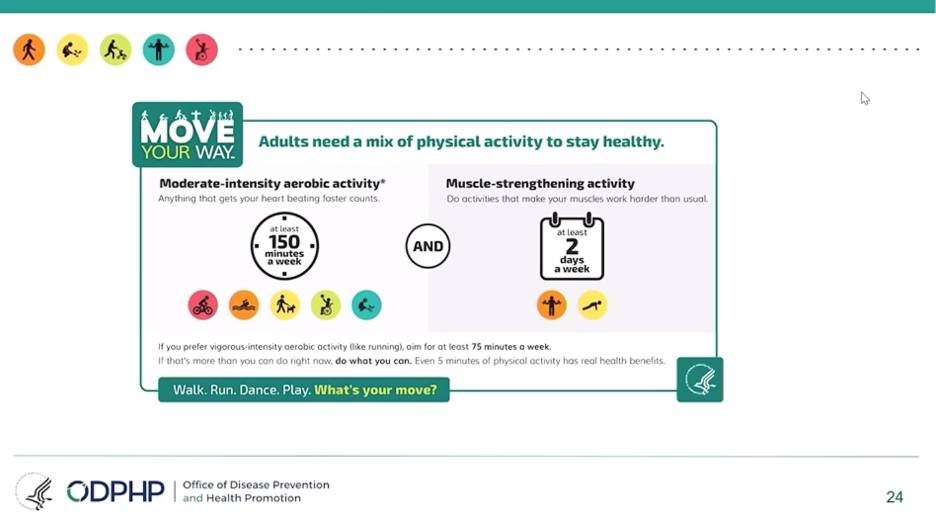
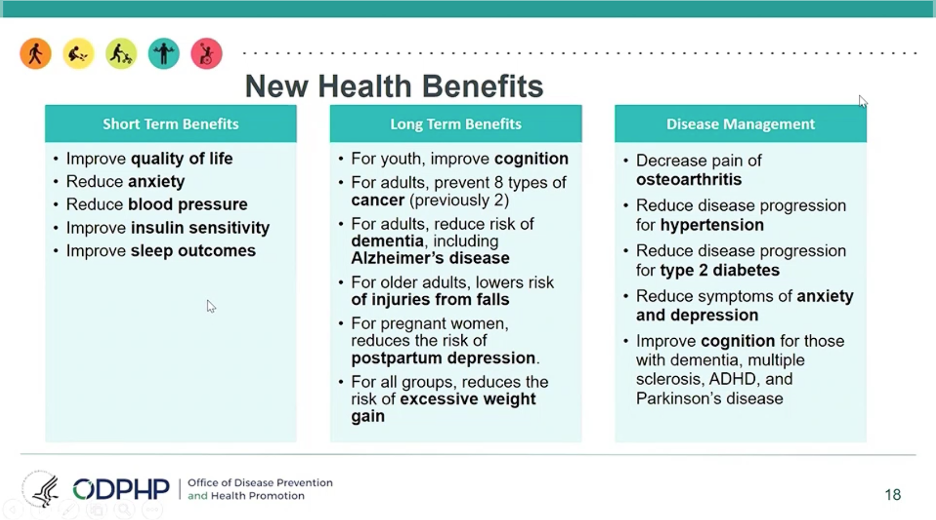
Adults (under age 55) should do at least 150 minutes (2.5 hours) to 300 minutes (5 hours) each week of moderate-intensity activity, or 75 minutes (1 hour and 15 minutes) to 150 minutes (2.5 hours) each week of vigorous-intensity aerobic physical activity. Adults should also do muscle-strengthening activities at least twice a week. We were slightly surprised not to see adults urged to exercise every day like former head of CMS/FDA Dr. David Kessler does in his recent acclaimed book, Fast Carbs, Slow Carbs.
-
Older adults (above age 55) should do the recommended aerobic and muscle-strengthening activities for adults. They should also incorporate balance and functional training, such as standing on one foot or ballroom dancing.
How do you determine the intensity of exercise? Dr. Piercy recommends the “talk test”: someone doing moderate-intensity aerobic activity can talk, but not sing, during the activity, while a person doing vigorous-intensity activity cannot say more than a few words without pausing for breath.
The speakers noted that while the most health benefits come with at least 150-300 minutes of moderate physical activity per week, any activity is beneficial: any time spent sitting that is swapped out for exercise (even light activity,) can lead to short-term and long-term health benefits. Read more about the guidelines here.
The Need for a Personalized Approach to Obesity Treatment
Experts shared the latest data on different treatments for obesity. They focused on three approaches:
1. Lifestyle interventions:
-
The Look AHEAD trial tested whether reducing calories and exercising regularly would lead to diabetes remission. After one year, 11.5% of participants achieved diabetes remission with an average weight loss of 19 pounds (8.6 kilos). After four years, 7.3% of participants were able to maintain remission with an average weight loss of 10 pounds (4.5 kilograms).
-
The Diabetes Remission Clinical Trial (DiRECT) tested whether calorie restriction alone had an effect on diabetes remission. After one year, 46% of people in this study with type 2 diabetes achieved remission; after two years, 70% of the people who had achieved remission were able to maintain remission.
Participants in Look AHEAD had more advanced diabetes than in DiRECT, leading to the big difference in remission rates. The speakers emphasized that the longer someone has been diagnosed with diabetes, the harder it is to achieve diabetes remission.
2. Obesity medication:
-
Just 2% of people living with obesity are managing the disease with medication. However, many obesity medications can lead to weight loss, prevention of diabetes, and diabetes remission.
-
Combination therapy has shown success for managing obesity and type 2 diabetes. A study testing tirzepatide (a dual GLP-1 and GIP receptor agonist) in people with type 2 diabetes found a 1.7-2% decrease in A1C and an average weight loss of 12 pounds in just 12 weeks.
3. Bariatric surgery:
-
Experts agreed that bariatric surgery should be considered as a treatment option for people with a BMI greater than 35. Bariatric surgery can also lead to sustained weight loss and a decrease in diseases associated with obesity, including sleep apnea and heart disease.
-
It’s clear that obesity treatments must be determined at individual levels – we know that so much more is possible for people with diabetes to reach healthier weights and will be returning to this topic. In the meantime, if changing your weight is of interest, talk to your doctor about how to do this in the best way for you.
Early CGM use can help kids and predict T1D progression
The use of CGM across different populations – including people of various ages and different stages of type 1 diabetes – shows that CGM can accurately predict the progression of type 1 diabetes for people at risk. For those transitioning from “stage 2” to “stage 3”, continuous monitoring can also help prevent DKA, which many people with type 1 have at diagnosis. While there are no clinical guidelines at the moment for how to manage “stage 2” type 1 diabetes, the TESS study is currently evaluating the benefits of CGM use in this population. “Staging” of type 1 diabetes is fairly new and we will be thinking about this more as we consider how to further improve education about type 1 diabetes.
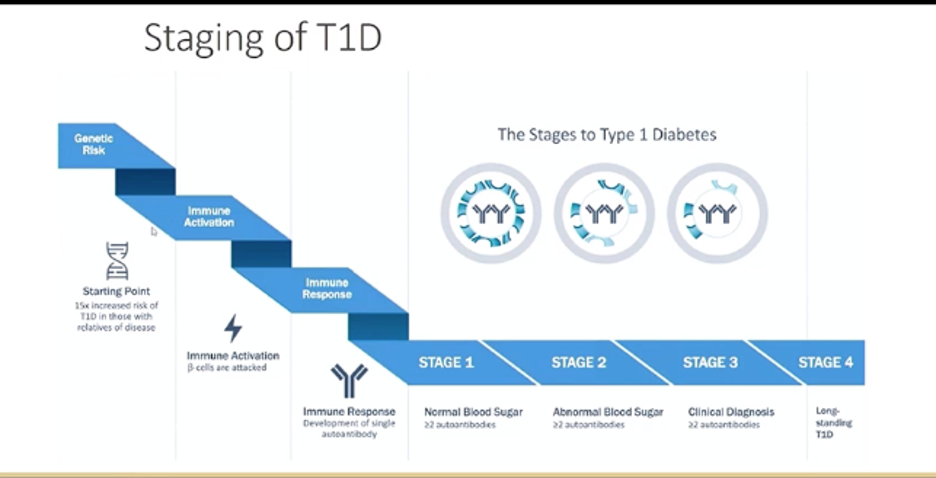
Experts all agreed that earlier use of CGM could result in better diabetes management later on. Dr. Jan Fairchild studied the start and continued use of CGM in a pediatric population with early “stage 3” type 1 diabetes. Kids who started CGM at diagnosis had slightly higher CGM wear at 24 months, compared to kids who started within the first two years of diagnosis (78% vs. 66%, respectively), though this result was not significant. All children using CGM ultimately benefitted – they demonstrated a median A1C of 7.7% at 24 months, which was less than the clinic median A1C of 8.1%. Dr. Fairchild also mentioned the educational role that early CGM use could play, especially with a focus on time in range.
The Debate on Metformin and Insulin Use During Pregnancy Continues
Traditionally, healthcare professionals have been advised to use insulin to treat pregnant women who have type 2 diabetes or gestational diabetes (GDM). Now, there is debate about whether metformin or other medications are equally effective alternatives to insulin.
Dr. Denice Feig presented data showing that in pregnant women with GDM, metformin use resulted in less maternal weight gain, less preeclampsia (pregnancy-related high blood pressure), lower birth weight, and less neonatal hypoglycemia (low blood sugar). Additionally, there is no evidence that metformin causes any abnormalities in babies, and the drug may reduce insulin resistance in the fetus. During the first trimester of pregnancy, metformin may be a reasonable alternative, if not a first-line treatment equivalent, to insulin. It is also cheaper, easier to use, and poses less of a risk for hypoglycemia (low blood sugar) than insulin.
While the data are promising, both Dr. Feig and Dr. Linda Barbour pointed out that long-term effects on the baby due to exposure to metformin during pregnancy may include a greater risk of being overweight, developing obesity, and having a higher BMI. Unfortunately, the data did not include pregnant women with type 2 diabetes; an ongoing study, MiTy, is currently studying these effects. Both Dr. Feig and Dr. Barbour emphasized that we need more data to decide the best treatment for pregnant women with diabetes – that may well be, and we also hope that better screening is in the works, so that those at risk of gestational diabetes can learn about it earlier and work with their healthcare teams to live with it successfully, which is eminently possible. Learn more about gestational diabetes in our recent article by Cheryl Alkon.








